How Many Valence Electrons Does a Neutral Magnesium Atom Have
Valency the Magnesium Mg. The elements that receive electrons and form bonds are called anions.

Energy Level Diagram For Magnesium Showing The Levels Relevant For This Download Scientific Diagram
The full number ofelectrons current in the valence covering of one atom are referred to as valence electronsand there room a total of two electrons present in the valence shell of magnesium 3s2.

. Posted on January 19 2022 By Rishad Hasan Cesium Atomic and Orbital Properties Cesium atoms have 55 electrons and the electronic shell structure is 2 8 18 18 8 1 with Atomic Term Symbol Quantum Numbers 2S12. How many valence electrons of beryllium ionBe 2 have. Magnesium is a neutral atom with 30 protons and 34 neutrons.
How many valence electrons does cesium ion have. Magnesium in its elemental form has 12 protons and 12 electrons. The neutral atom will have 12 electrons around it.
The elements that have 5 6 or 7 electrons in the last shell orbit receive the electrons in the last shell during bond formation. It has two free valence electrons which are used to bond with other atoms that have two valence electrons commonly oxygen or nitrogen. In this case both the valency and valence electrons of beryllium are 2.
Magnesium has a atomic number of 12 and has a total number of 12 electrons. Magnesium has two valence electrons. 1s22s22p63s23p4 how many valence electrons does this atom have.
We know the details about this. Hence n 3 principal energy level for 3s and l 0 for s orbital. That means there are 12.
When they produce ionic compounds the majority of nonmetals become anions. 2 4 6 12. Electrons in an atom of magnesium.
There are 10 electrons in an Mg2 ion. After the electron configuration the last shell of the beryllium atom has two electrons. 11 How many 4p electrons are in GE.
This completely fills the 1st and 2nd electron shells. Magnesium is a form 12 and is classified in Group 2 of the Periodic Table. Analysed by bank level 1.
Magnesium has 2 valence electrons. Lithium has a single electron in the second principal energy level and so we say that lithium has one valence electron. This electron configuration shows that iron ion Fe 2 has three shells and the last shell has fourteen electrons.
15 How many valence does El have. Hydrogen has a 1 charge. The sodium atom has 11 protons 11 electrons and 12 neutrons.
13 How many valence electrons does xenon have. Magesiums average atomic mass is 24305 atomic mass units but no magnesium atom has exactly this mass. The Mg2 ion is formed when the neutral magnesium atom loses 2 electrons which brings its total number of electrons to 10.
An Na ion is a sodium atom that has lost one electron as that makes the number of electrons in the atom equal to that of the nearest Nobel gas Neon which has 10 electrons. The neutrons are a different matter. How many valence electrons are in an atom of.
Carbon has four empty spaces in its outer shell enabling it to bond to four other atoms. Indium atoms have 49 electrons and the shell structure is 28. Visit the simulation Covalent bonding between hydrogen atoms.
We know the details about this. A magnesium mg atom has a total of twelve electrons. A neutral Phosphorus Atom has five valence electrons.
The ground state electron configuration of ground state gaseous neutral indium is Kr. Fe 3e Fe 3. Also the electron configuration of Mg is 1s² 2s²2p⁶ 3s² or Ne3s².
Jan 13 2015. The element magnesium has the atomic number 12 and it will have 12 protons in its nucleus. A neutral atom of magnesium would have 12 electrons to balance out the positive charge of the 12 protons found.
How Many Valence Electrons Does A Magnesium. Analyst bank clerk bank po. Valency of Magnesium Mg.
It an atom has 12 electrons and is neutral then it must be Mg magnesium and magnesium has 2 valence electrons which would be used in bonding. How many electrons does magnesium 25 have. Again the iron atom donates two electrons in 4s orbital and an electron in 3d orbital to convert iron ion Fe 3.
Mg s O2 g MgO s or simply 2. Furthermore how many neutrons does mg2 have. How many electrons does indium have in a neutral atom.
In your talk remember to speak about. How many valence electrons does indium in have. The electron configuration of phosphorus through the sub-orbit is 1s 2 2s 2 2p 6 3s 2 3p 3.
Since the 3s² electrons are the outermost electrons magnesium has two valence electrons. The sodium ion only has 10 electrons and a 1 charge on the right. For this iron ion Fe 2 has a total of fourteen valence electrons.
It require one more electron to. 10 How many energy levels and valence electrons does an atom of germanium Ge have. For the main group elements not transition metals the group number tells us the number of valence electrons in.
They differ only because a 24 Mg atom has 12 neutrons in its nucleus a 25 Mg atom has 13. The number of electrons per shell of phosphorus is 2 8 5. The total number of electrons present in the valence shell of an atom are called valence electrons and there are a total of two electrons present in the valence shell of magnesium 3s2.
Mgs electron configuration is also known as Ne3s- Does Magnesium Have 12 Electrons. Magnesium is element 12 and belongs to Group 2 of the Periodic Table. To obtain an octet in chlorines.
Electron Configurations Watch on. Magnesium has 2 valence electrons and thus it would rather give those up. Thusmagnesium has actually two valence electrons.
A sodium atom has 11 electrons on the left. In its outermost shell a neutral chlorine atom has seven electrons. Magnesium has two valence electrons.
23 hours agoHelium is located in period 1 group 18 of the Periodic Table and has an atomic number equal to 2. 8 Why does C have 4 valence electrons. 12 How many neutrons does GE have.
9 How many 4s electrons are in GE. In this case the valence electrons of nitrogen are 5. Two valence electrons are present in Group 2s element.
Which means it has 7 electrons in its valence shell. Note that magnesium will loan out electrons to form. How many valence electrons does a neutral atom of lithium have.
Thus magnesium has two valence electrons. Magnesium in its elemental form has 12 protons and 12 electrons. It is 11070 c 1380.
That is the first shell of phosphorusP has two electrons the second shell has eight electrons and the 3rd shell has five electrons. An element in Group 2 has two valence electrons. Magesiums average atomic mass is 24305 atomic mass units but no magnesium atom has exactly this mass.
Electrons so this level only has two electrons total. Magnesium has a atomic number of 12 and has a total number of 12 electrons. 14 What is the orbital notation of GE.
A neutral atom of indium will have three valence shell electrons. Helium has 2 electrons in its outer electron shell so 2 dots. So magnesium is element number 12 in the periodic table.
The Number Of Electron Levels In A Magnesium Atom Is At Level

Write A Complete Electron Configuration For A Neutral Magnesium Atom Enter Your Answer Using This Format Homeworklib

How Many Valence Electrons Does Argon Ar Have

How To Draw The Bohr Rutherford Diagram Of Magnesium Youtube

The Number Of Electron Levels In A Magnesium Atom Is At Level

1 Ionic And Metallic Bonding Ch Review What Is A Valence Electron Electrons In The Highest Outermost Occupied Energy Level Related To The Group Ppt Download

The Number Of Electron Levels In A Magnesium Atom Is At Level

3 433 Gostos 24 Comentarios Vanessa Adagio Studies No Instagram Managed To Get Stuff Done Today Notes Inspiration School Organization Notes Study Notes

Magnesium Bohr Model How To Draw Bohr Diagram For Magnesium Mg
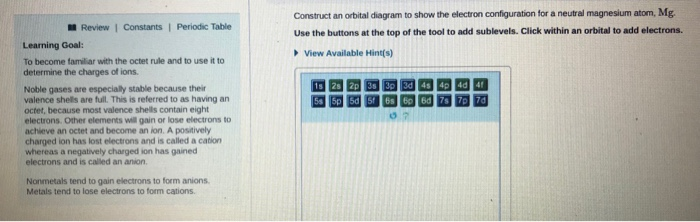
Solved Construct An Orbital Diagram To Show The Electron Chegg Com

Ions Big Idea Atoms Can Gain Or Lose Electrons To Form Charged Particles Called Ions Ppt Download

Webelements Periodic Table Magnesium Properties Of Free Atoms

How Many Valence Electrons Does Magnesium Mg Have Valency Of Magnesium
How Many Electrons Does Magnesium Have Quora

How Many Valence Electrons Does Magnesium Mg Have
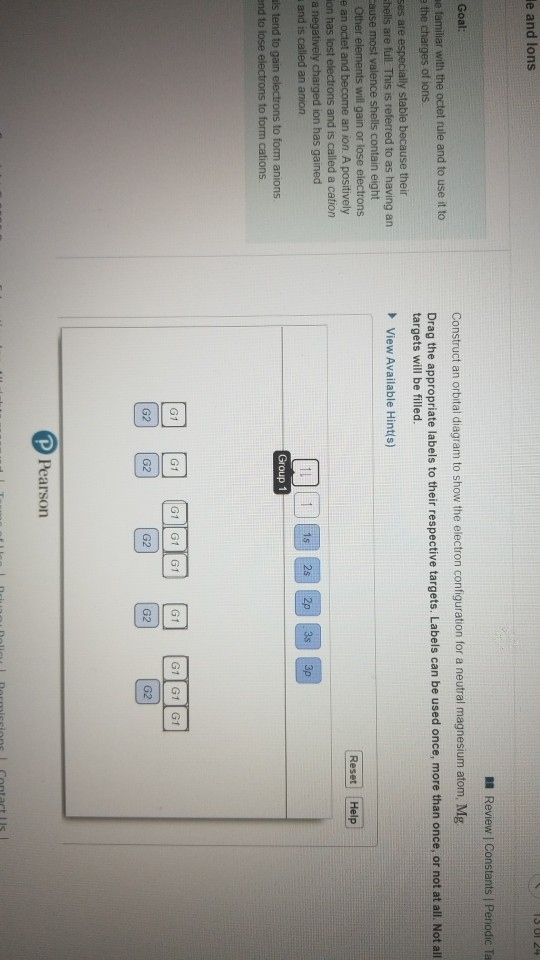
Solved Is Ul24 Le And Lons A Review Constants Periodic Ta Chegg Com
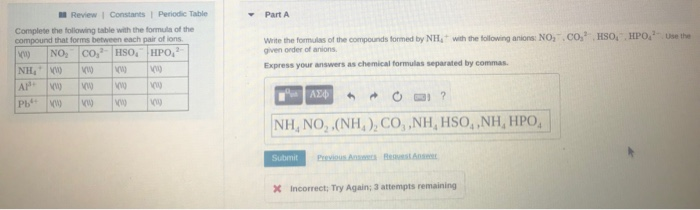
Solved Construct An Orbital Diagram To Show The Electron Chegg Com

The Atom What Is It Made Of Protons Positively Charged Mass 1 Amu 1 67 X Grams Located In The Nucleus Gives An Atoms Its Identity Ppt Download

Chemistry Review You Need To Remember Some Basic Things Ppt Download
Comments
Post a Comment